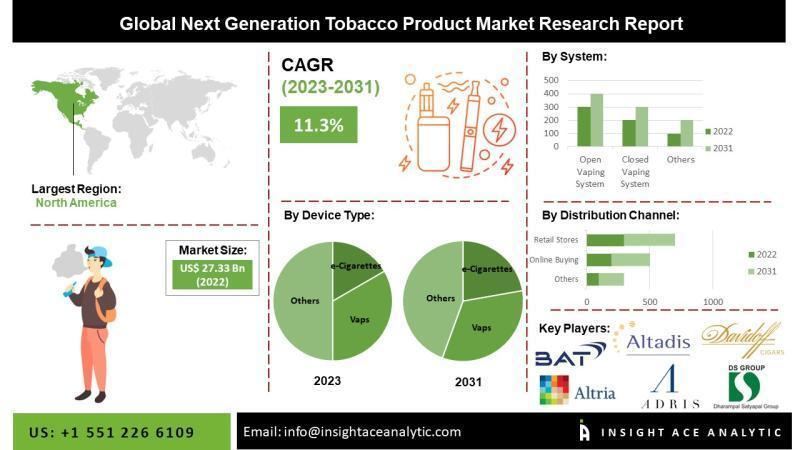
According to a report by openpr on May 28th, a new study by InsightAce Analytic predicts that the global Next Generation Tobacco Product market will reach a market value of $673.8 billion by 2030. These products include e-cigarettes, vapes, mods, e-hookahs, pens, and other devices.
InsightAce Analytic has just released a global assessment report on the next generation tobacco products market. The report highlights that this type of product includes open, closed, semi-closed systems, as well as other tobacco heating, vaporizing, and smokeless tobacco products. Components include vaporizers, vapor modulators, vaporizer cartridges, e-liquid, batteries, and other accessories. Products can be distributed through online purchases, retail stores, convenience stores, pharmacies, newsstands, tobacco shops, and specialized e-cigarette stores.
In 2021, the global next generation tobacco products market is expected to reach $248.3 billion, with a projected continuous growth at a compound annual growth rate of 11.8% from 2022 to 2030. Nicotine, the main component of tobacco products, is a key factor in preventing smokers from quitting. The World Health Organization (WHO) estimates that over 8 million people die each year due to smoking. To address this issue, tobacco product manufacturers are implementing cutting-edge strategies to reduce the likelihood of tobacco-related diseases.
With more products entering the market, the demand for next-generation tobacco products is increasing. During the forecast period, the expansion of next-generation tobacco products will also be significantly influenced by the availability of high-quality goods. Due to rapid product development and commercialization, including next-generation tobacco products such as smokeless tobacco, vaping products, and tobacco heating devices, the demand is expected to continue to grow throughout the forecast period.
According to predictions, investment in the next generation of tobacco products is expected to significantly increase in the coming years, with growing consumption among women and students. The next generation tobacco products market is currently witnessing substantial demand growth due to the increase in disposable income among consumers. Rapid improvement in consumer knowledge and product awareness, as well as changing preferences for less harmful tobacco products, are also expected to provide significant growth opportunities for the next generation tobacco products industry.
In the forecasted years, the Asia-Pacific region is expected to make significant contributions to the next generation tobacco products market due to various factors, including increased government investment in tobacco product research and development, improved public awareness of these products, rising quit rates, and high demand for e-cigarettes and vaporizers. Furthermore, with increased investment in future technologies, growing demand for tobacco products, and an increase in smoking-related illnesses, the North American next generation tobacco products market is expected to experience significant growth during the anticipated period.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







