
Special statement:
This article is for internal industry communication only and does not make any recommendations for specific brands or products.
The images presented in this article are only used to describe the facts and are not intended as advertisements for any products.
Minors are prohibited from accessing this article.
Key Takeaways:
- Regulatory milestone: ON!® PLUS has passed FDA PMTA review and received a Marketing Granted Order (MGO), entering the list of nicotine pouch products authorized for legal sale in the United States.
- Product configuration: All six authorized products feature a Slim pouch format with FlexTech elastic pouch material, contain 20 pouches per can, and are limited to 6 mg and 9 mg nicotine strengths.
- Flavour strategy: Authorized flavours focus on Mint, Wintergreen, and Tobacco, reflecting a conservative flavour structure aligned with current U.S. regulatory expectations.
2Firsts, December 22, 2025 — On December 19, 2Firsts observed that the U.S. Food and Drug Administration (FDA) updated its official list of “Nicotine Pouches Authorized Products,” disclosing that ON!® PLUS nicotine pouch products have successfully passed Pre-Market Tobacco Product Application (PMTA) review and received Marketing Granted Orders (MGOs), allowing them to be legally marketed in the United States.
Related Reading:
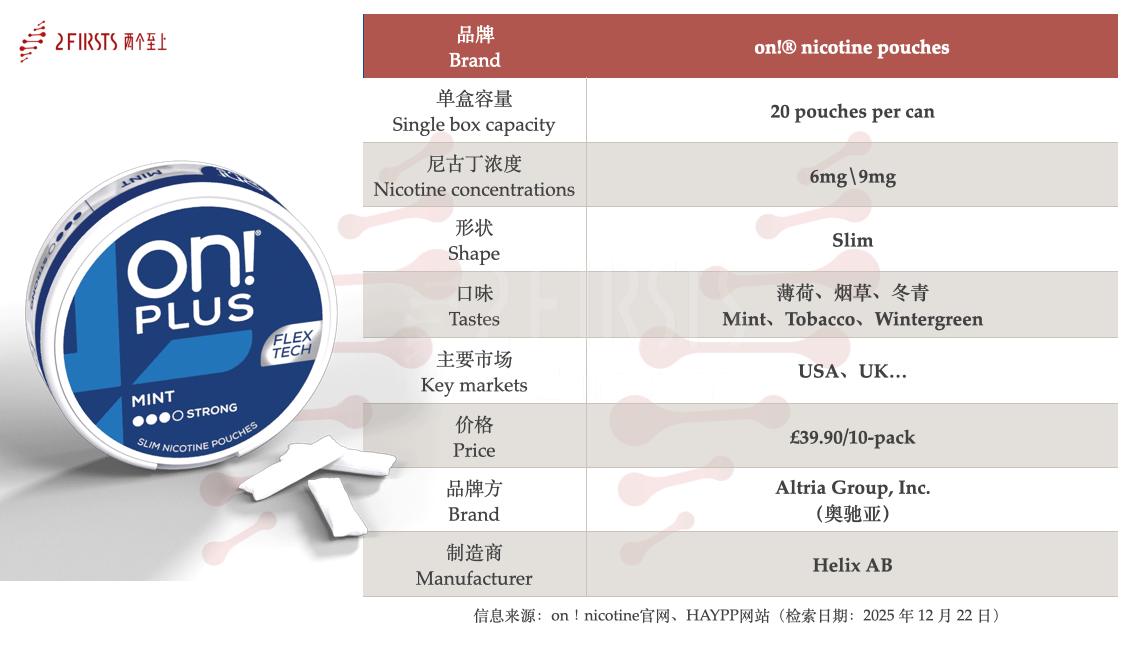
Based on the FDA’s latest authorization disclosures, 2Firsts reviewed information from the official on!® website and publicly available sources to systematically organize the core specifications and parameters of the six ON!® PLUS nicotine pouch products that have passed PMTA review and received MGOs, as detailed below.
Graphic: 2Firsts
Two Nicotine Strengths (6 mg / 9 mg) Three Traditional Flavours: Mint, Wintergreen, Tobacco
According to the authorized product specifications, all six ON!® PLUS nicotine pouch products adopt a Slim pouch format and utilize FlexTech (Flexible Pouch Technology) elastic pouch material, with 20 pouches per can. In terms of nicotine strength, the products are limited to 6 mg (Regular) and 9 mg (Strong) variants.
From a flavour perspective, the six MGO-authorized products are concentrated in Mint, Wintergreen, and Tobacco—traditional flavour categories. Overall, the flavour structure is relatively conservative, aligning with the mainstream acceptance range under the current U.S. regulatory framework for nicotine pouches.
Channel information indicates that ON!® PLUS nicotine pouches are manufactured by Helix AB (Sweden) and commercially operated in the U.S. market by Altria Group, Inc. The primary sales markets include the United States and the United Kingdom, with distribution also extending to other European countries.

Image source: Helix AB
According to Helix Sweden AB’s official website, the retail price of on!® PLUS in the UK market is £39.9 per 10 packs.
ON!® PLUS Product Line:Up to 11 mg and Multiple Mixed Flavours in the UK Market
According to the official on!® product pages, the ON!® PLUS product line offers a more comprehensive range of strengths and flavours in the UK market. Nicotine strength options include 6 mg (Regular), 9 mg (Strong), and 11 mg (Extra Strong), with 11 mg representing the highest strength in the series and available only in selected flavours.
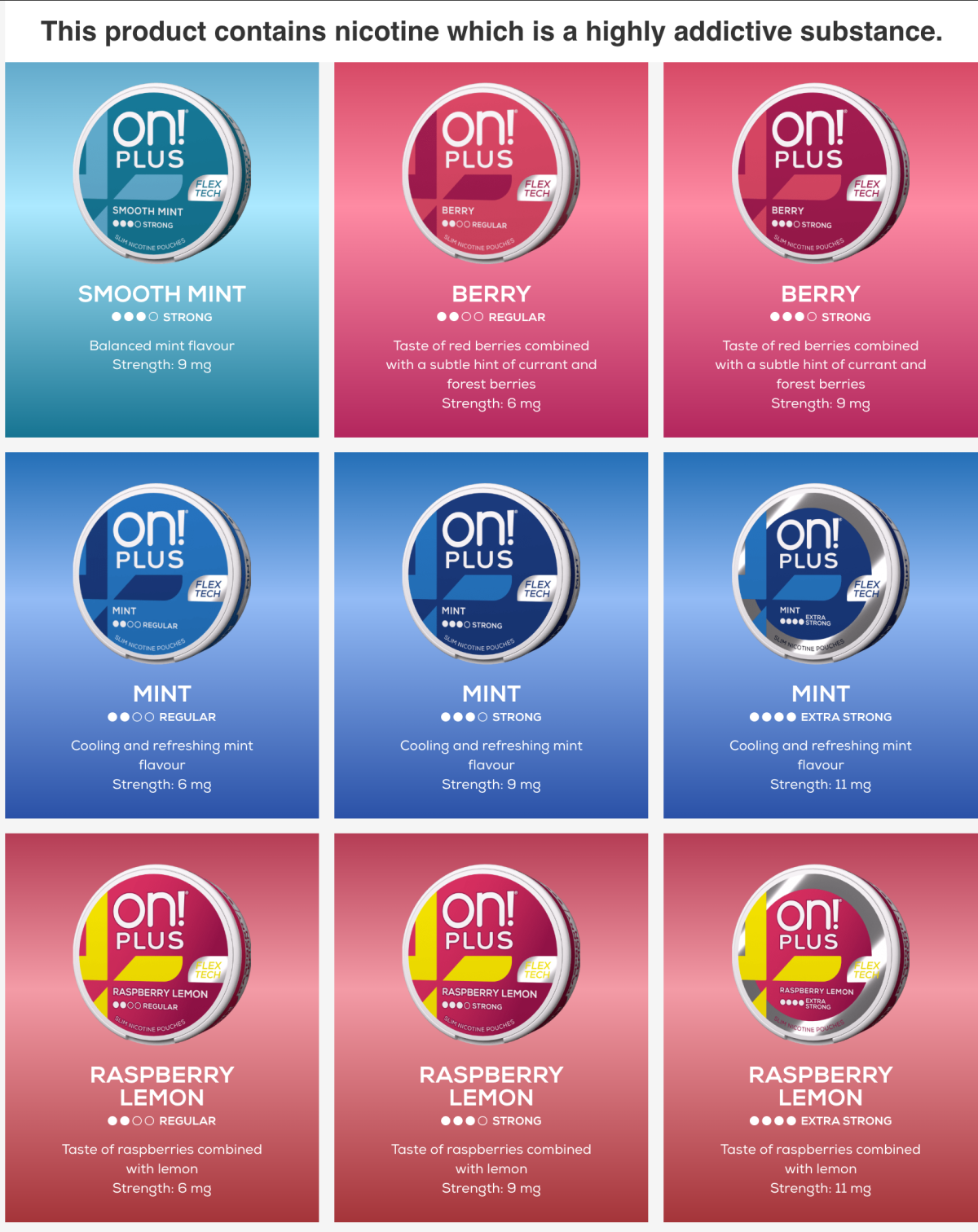
Image source: on!® official website
In terms of flavours, the UK market extends beyond traditional options and includes:
- Mint
- Smooth Mint
- Citrus (grapefruit, lime, and citrus blend)
- Berry (red berry profile)
- Raspberry Lemon
- Watermelon Mint
Overall, most flavours are offered in Regular and Strong strengths, with selected variants extending to Extra Strong.
on!® mini:A Differentiated Product Line Featuring Ultra-Small Size and Dry Pouch Design
In addition to ON!® PLUS, the on!® brand also showcases the on!® mini nicotine pouch line on its official website. This series emphasizes an ultra-small format and high discreteness, and is positioned by the brand as one of the smallest nicotine pouch products currently available on the market.

Image source: on!® official website
Unlike ON!® PLUS, which is described as a “moist slim nicotine pouch,” the on!® mini series adopts a dry pouch design, featuring a more compact pouch structure.
As a leading global NGP media and think tank, 2Firsts is dedicated to providing the latest product and technological information and insights to practitioners around the world in various categories such as e-cigarettes, heated tobacco products, and modern oral products. It aims to drive technological changes and innovations in NGP products worldwide, thereby offering tobacco consumers globally with safer products and lifestyles.
With a source of information covering the supply chains in China and global markets, 2Firsts product coverage has become one of the most influential platforms for new product and technology releases globally.
Contact 2Firsts for the following services:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Identifying sales channels for products.
Contact Information: Email: info@2firsts.com
Contact CEO Alan Zhao of 2Firsts on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







