
Key Points:
- Ban Decision: FDA issued an MDO against Imperial Tobacco’s blu Disposable 2.4% Classic Tobacco e-cigarette, prohibiting sales and distribution in the US.
- Core Reason: FDA concluded that the application lacked sufficient scientific evidence to demonstrate that the product’s public health benefits outweigh its risks.
- Health Concerns: Evidence showed users may continue smoking while using the product (“dual use”), leading to exposure to harmful substances at levels comparable to or greater than smoking alone.
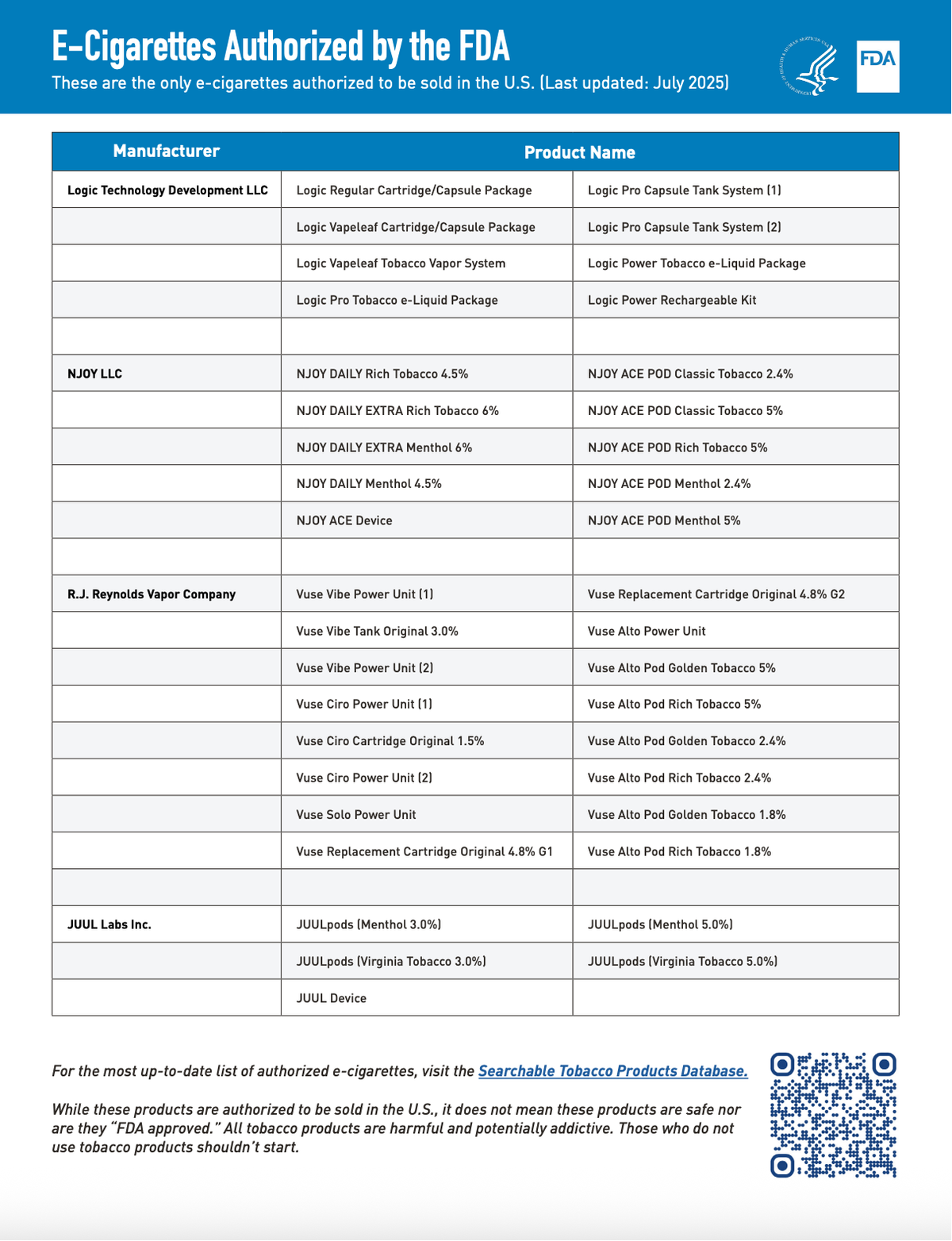
- Regulatory Context: Under the Family Smoking Prevention and Tobacco Control Act, all e-cigarette products must undergo FDA review and receive authorization before legal sale. To date, 39 e-cigarette products and devices have been authorized, excluding blu.
- Next Steps: blu may submit a new application for marketing authorization. Until approval is granted, the product cannot be sold in the US, and manufacturers, distributors, and retailers risk enforcement actions for violations.
According to an FDA announcement on August 19, the agency has issued an MDO for the blu® Disposable Classic Tobacco 2.4%, produced by Imperial Tobacco subsidiary Fontem US, LLC. This means the company is still prohibited from selling or distributing the product in the US market unless a new application is submitted and authorized.

The FDA evaluates Premarket Tobacco Product Applications (PMTAs) based on a public health standard, which considers the risks and benefits of a product to the entire population. After reviewing the company’s PMTA, the FDA determined that the application lacked sufficient evidence to demonstrate that authorizing this product would meet the requirement of protecting public health — the statutory standard set by the 2009 Family Smoking Prevention and Tobacco Control Act.
The company failed to provide adequate evidence showing that smokers would completely switch to this new product or significantly reduce their cigarette consumption. On the contrary, the evidence in the application suggested that people were more likely to use the product while continuing to smoke cigarettes. This could potentially expose them to higher levels of toxic substances than smoking cigarettes alone. In general, long-term concurrent use of e-cigarettes and cigarettes — commonly referred to as “dual use” — may pose health risks comparable to, or even greater than, smoking cigarettes alone.
In contrast, the FDA has authorized certain e-cigarettes currently available on the market because evidence shows that smokers either completely switch to these products or significantly reduce their cigarette use, with the potential for lower overall harm.
Dr. Bret Koplow, Acting Director of the FDA’s Center for Tobacco Products, stated:
“While FDA-authorized e-cigarettes are a less harmful alternative for smokers — especially when they completely switch — not all e-cigarettes are the same. FDA’s rigorous scientific review ensures that authorized e-cigarettes provide a net benefit to public health. In this case, the company did not provide sufficient evidence to show that the benefits of its product outweigh the risks, particularly since the evidence indicates that smokers typically do not quit or significantly reduce cigarette use while using this product.”
The FDA emphasized that tobacco products subject to a Marketing Denial Order (MDO) may not be introduced or delivered for introduction into interstate commerce and must be removed from the market. Manufacturers, distributors, and retailers who sell or distribute such products in interstate commerce are in violation of the law and will face enforcement actions. Information about MDOs is published on FDA’s Tobacco Product Marketing Orders webpage.
According to the FDA, the August 19 action is part of the agency’s ongoing effort to ensure that all new tobacco products sold in the U.S. undergo scientific review and receive marketing authorization. To date, the FDA has authorized 39 e-cigarette products and devices; these are the only e-cigarettes legally permitted to be sold and distributed in the United States. For a searchable list of tobacco products that can be legally sold and distributed in the U.S., please visit the FDA’s “Searchable Tobacco Products Database.”
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







