
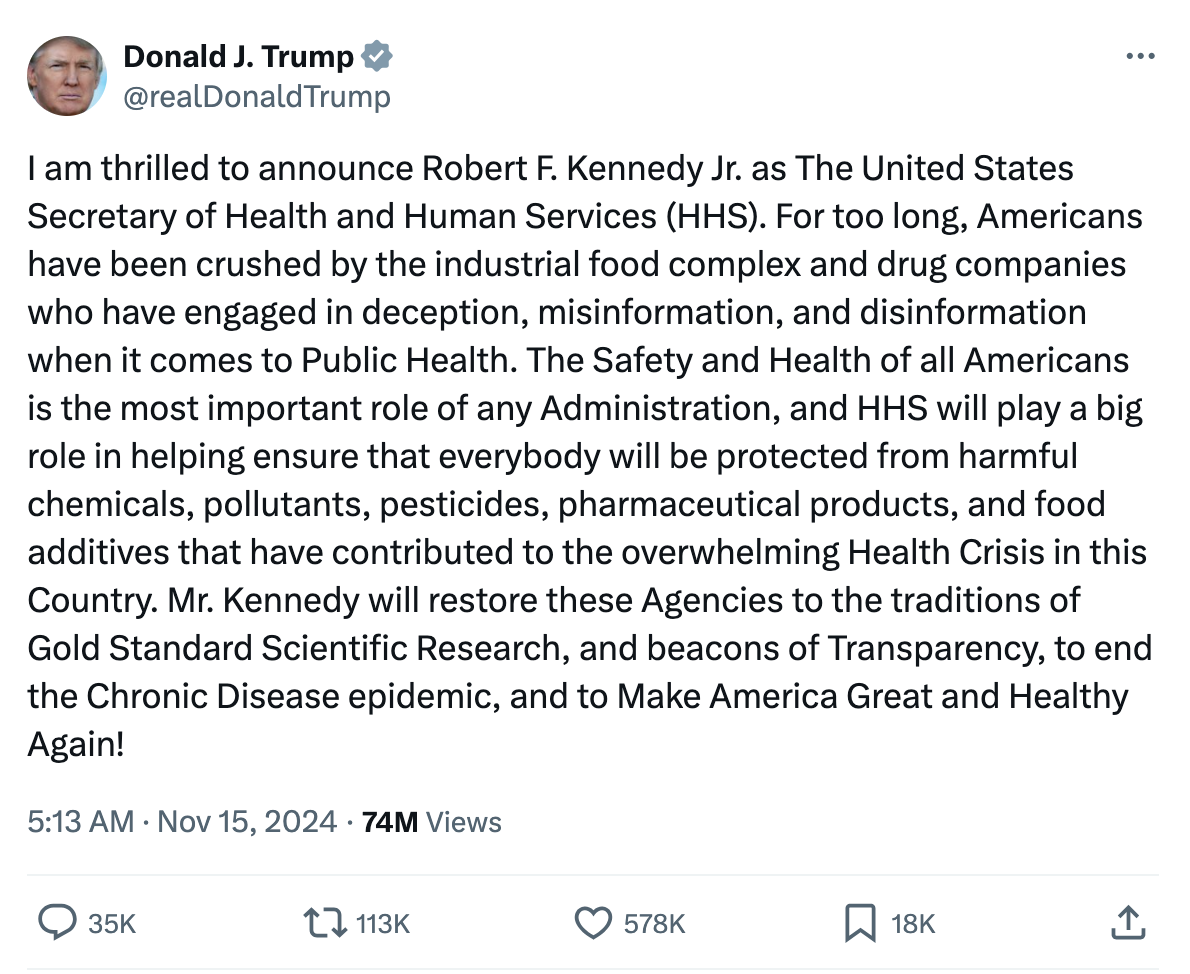
According to Vaping360, on November 15th, newly elected U.S. President Donald Trump announced his nomination of Robert F. Kennedy, Jr. as the Secretary of the Department of Health and Human Services (HHS).
"For too long, Americans have been crushed by the industrial food complex and drug companies who have engaged in deception, misinformation, and disinformation when it comes to Public Health," Trump post on social media.
"The Safety and Health of all Americans is the most important role of any Administration, and HHS will play a big role in helping ensure that everybody will be protected from harmful chemicals, pollutants, pesticides, pharmaceutical products, and food additives that have contributed to the overwhelming Health Crisis in this Country."
As the leader of the HHS, Kennedy will oversee FDA, which regulates vapes, nicotine, and tobacco products.
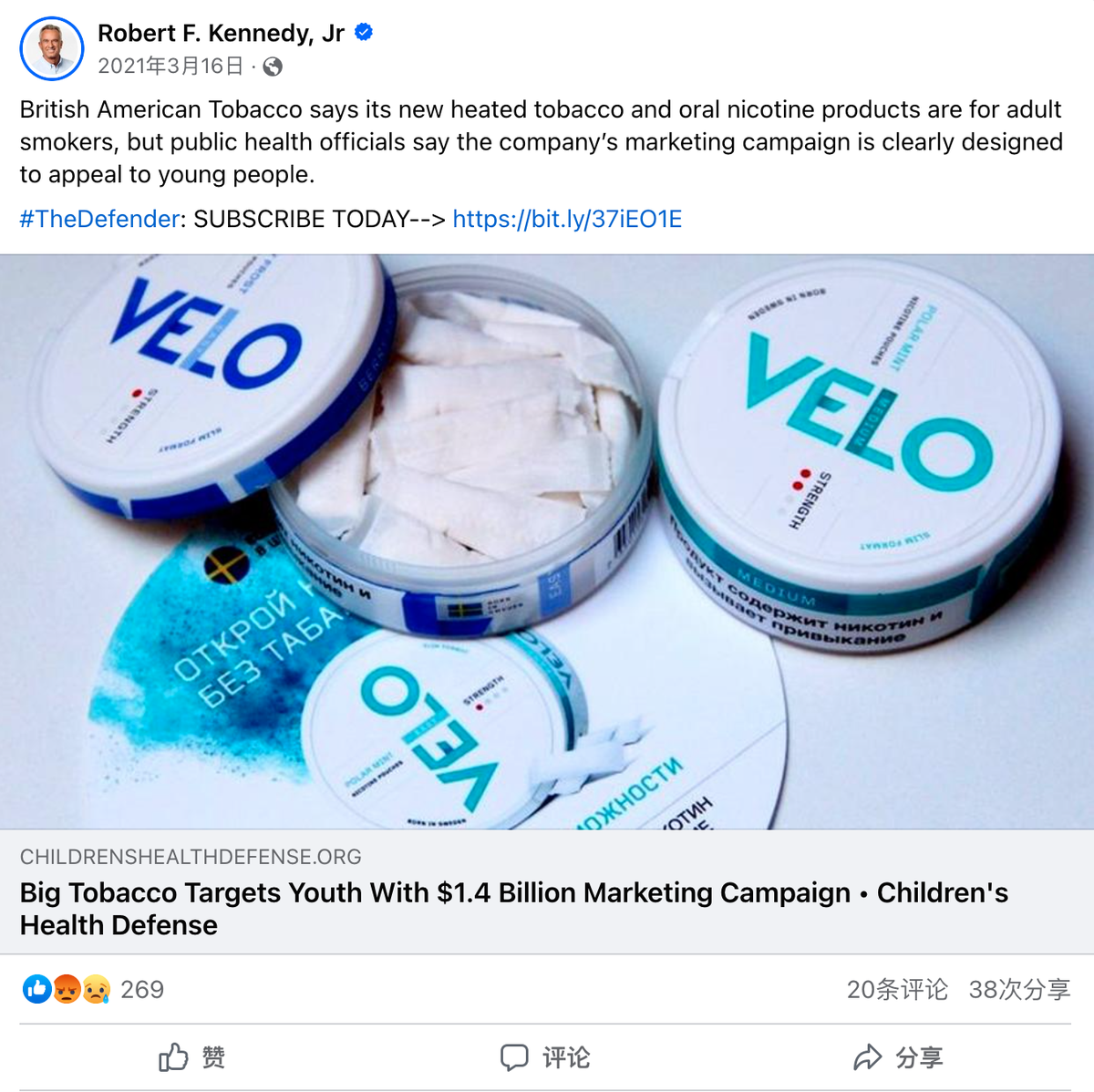
In March 2021, Kennedy criticized BAT for selling vapes and smokeless nicotine products targeted at children on his Facebook page. There is little information about his position on tobacco regulation. It is unclear where Kennedy stands on issues related to vapes, nicotine, and tobacco harm reduction.
During the presidential campaign, Trump promised that, if elected, he (Trump) would "save vaping".
If confirmed by the Senate, Kennedy would become the Secretary of HHS, overseeing key agencies like the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), and the Centers for Medicare and Medicaid Services (CMS), which administers the majority of the nation’s public healthcare programs. HHS manages a budget exceeding $1 trillion.
"He wants to do some things, and we’re going to let him get to it," Trump said in his election victory speech. "Go have a good time, Bobby."
This year at age 70, Kennedy is the son of former Senator and Attorney General Robert F. Kennedy, as well as the nephew of former President John F. Kennedy, both iconic figures of the Democratic Party. As a seasoned environmental activist and lawyer, Kennedy first ran for president as a Democrat, then as an Independent, but later dropped out and endorsed Trump in exchange for influence on health and agriculture policies in Trump's second term.
President Trump stated that he would allow Kennedy to "go wild," as Kennedy is known for his views on vaccines causing autism. He mentioned that the new Trump administration will oppose the addition of fluoride to drinking water, ban certain foods and additives, and promote the consumption of unpasteurized "raw" milk, which has been linked to e-coli.
Kennedy will face Senate confirmation hearings sometime after Trump is sworn in as President in late January.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







