
Key Points:
·Rising Cases: From 2022 to 2025, there has been an increase in cases of nicotine pouch exposure reported to the American Poison Control Center, with 72% involving children under the age of 5.
·Health Risks: Even a small amount (1-4mg) can be toxic, leading to symptoms such as confusion, vomiting, and loss of consciousness.
·FDA Concerns: The fruity flavors and bright packaging of these products resemble candy, making them appealing to children.
·Protective Measures: The FDA is urging manufacturers to use child-resistant packaging and for parents to store these products safely out of reach of children.
On September 3, 2025 - According to a message posted on the FDA's official website on September 2, the U.S. Food and Drug Administration (FDA) is urging nicotine pouch manufacturers to use child-resistant packaging to protect American children from accidental harmful exposure. The number of reported nicotine pouch exposure cases has been steadily increasing from April 1, 2022, to March 31, 2025, as reported by the American Association of Poison Control Centers. Approximately 72% of nicotine pouch exposure cases occurred in children under the age of 5.
The FDA stated that nicotine pouches contain high concentrations of nicotine, which can be harmful or even fatal to children even in small amounts. Reports have indicated that doses of 1 to 4 milligrams of nicotine can have toxic effects on children. Symptoms of nicotine poisoning may include confusion, vomiting, and loss of consciousness.
I am concerned about the increasing reports of children being exposed to nicotine through nicotine pouches," said FDA Commissioner Marty Makary. "The fruity flavors and vibrant colors of nicotine pouch products may resemble candy and be attractive to children. Manufacturers should consider taking measures to prevent accidental exposure and ingestion.
The FDA has also issued information on how to properly store nicotine pouches to prevent accidental exposure to children. Parents and caregivers should store all nicotine products, including nicotine pouches, securely in their original packaging out of reach of children, and seek medical attention immediately if accidental ingestion occurs. If anyone ingests a nicotine pouch by mistake, they should call the Poison Control Hotline at 1-800-222-1222 immediately.
The FDA states that child-resistant packaging is a key safety barrier to prevent children from accidentally ingesting nicotine products, and also reflects manufacturers' commitment to protecting public health. Manufacturers of nicotine pouch products intending to adopt child-resistant packaging or other measures to reduce the risk of children's accidental exposure should contact the FDA through a designated regulatory health project manager or the email AskCTP@fda.hhs.gov, or by phone at 877-CTP-1373.
The FDA also stated that they have authorized 20 varieties of nicotine pouch products that use child-resistant packaging.
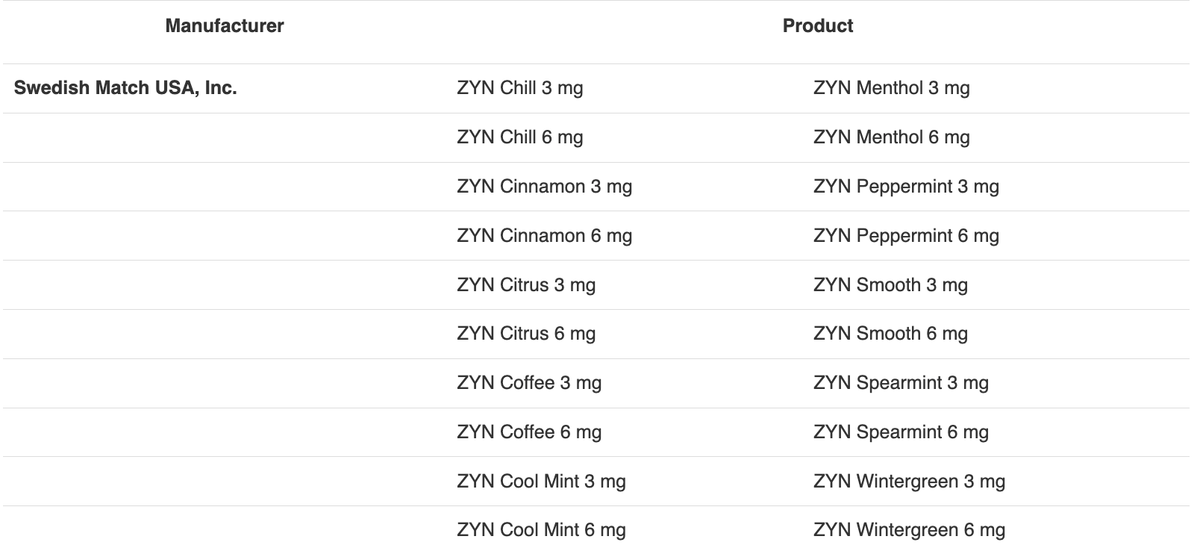
2Firsts noted that all authorized nicotine pouch products belong to the ZYN brand, produced by Swedish Match Company, a subsidiary of Philip Morris International (PMI).
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







