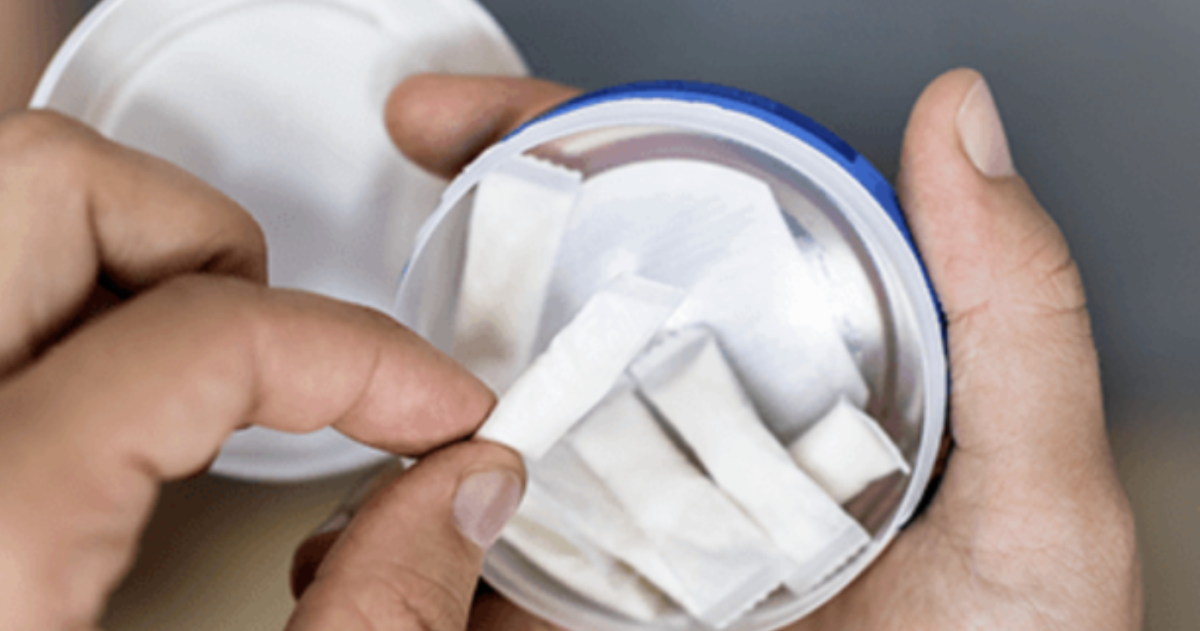
Key Points:
·The main content of the standard includes: limited food-grade or pharmaceutical-grade ingredients, with a nicotine content ≤16.6 milligrams per pouch. It requires GMP certification for production and complete traceability of raw materials. Toxicological risk assessment is mandatory. It also stipulates tamper-proof packaging and complete ingredient labeling.
·Industry significance: The first specific standard for nicotine pouches in France. It integrates mature regulatory experience from the UK and Sweden. It aims to curb the circulation of illegal products in the market.
According to an announcement on July 2 on the official website of BAT France (British American Tobacco France), the French Standardization Association AFNOR recently released an experimental standard XP V37-500, which sets specific requirements for nicotine pouch standards. The standard was developed with the participation of experts, manufacturers, evaluators, and research institutions, marking an important step forward in the industry's structuring process. BAT France stated that they are satisfied with the standard.
According to reports, the French Standardization Association (AFNOR) is the official standardization organization in France and is the government-authorized national body responsible for standardization.
The specific requirements for XP V37-500 are as follows:
·Product ingredients: Only food-grade or pharmaceutical-grade ingredients are allowed. Nicotine must be of natural origin, meet standard quality specifications, and contain no more than 16.6 milligrams per bag.
·Manufacturing: Must follow strict Good Manufacturing Practices (GMP) and ensure full traceability of raw materials.
·Toxicology assessment: Each ingredient must undergo Toxicological Risk Assessment (TRA), including potential impurities, packaging materials, and consumer contact time.
·Packaging: Materials that come into direct or indirect contact with the product must meet European standards for food contact and tamper-evident packaging.
·Labels: Must inform consumers of nicotine content, ingredients, the presence of allergens or skin sensitizers, and usage precautions. Additionally, consumers must be clearly warned of the addictive nature of nicotine.
The XP V37-500 standard, based on current standards in the UK and Sweden, draws on effective practices at the European level to provide a reliable, coordinated, and immediately implementable framework. This standard reflects the tobacco industry's desire to self-regulate and combat the proliferation of unregulated products on the market in order to better protect adult smokers.
The Public Affairs Director of British American Tobacco France, Sébastien Charbonneau, stated:
"Standards like XP V37-500 provide a quality mark, reliable quality assurance, and transparency for products targeted at adult consumers sold through professional networks. This is another step towards stricter regulation of nicotine pouch e-cigarettes and cracking down on the uncontrolled illegal market."
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







