
Key points:
1. Smoore's brand MOYAL Lan Zhi has obtained a Class II medical device certificate. Its high-end medical device project will settle in the Hezhou High-tech Zone in Guangxi in September 2024, and a production ceremony will be held on February 26, 2025.
2. The high-end medical equipment project in Hezhou focuses on the production of advanced medical devices such as atomizing beauty equipment, with an expected annual revenue of over 1 billion yuan ($138 million) within 3 years.
3. Pusimei Health operates multiple subsidiaries, with the ultimate controlling entity being Pusimei Hong Kong Limited. One of its subsidiaries, Shenzhen Moer Atomization Health Medical Technology Co., Ltd., has registered the "Lanzhi" brand trademark under 11 categories.
Smoore Technology said on March 4 that its skincare brand MOYAL has obtained a Class II medical device certificate and held a "Non-invasive High-end Medical Device Project Commissioning Ceremony" in the Hezhou High-tech Industrial Development Zone, Guangxi.
Wu Junlan, Vice President and President of the Beauty Division of Smoore International Holdings Limited, said: "The new factory will fully take over the production and manufacturing of medical devices on the MOYAL Lan to TPS technology platform," according to the article.
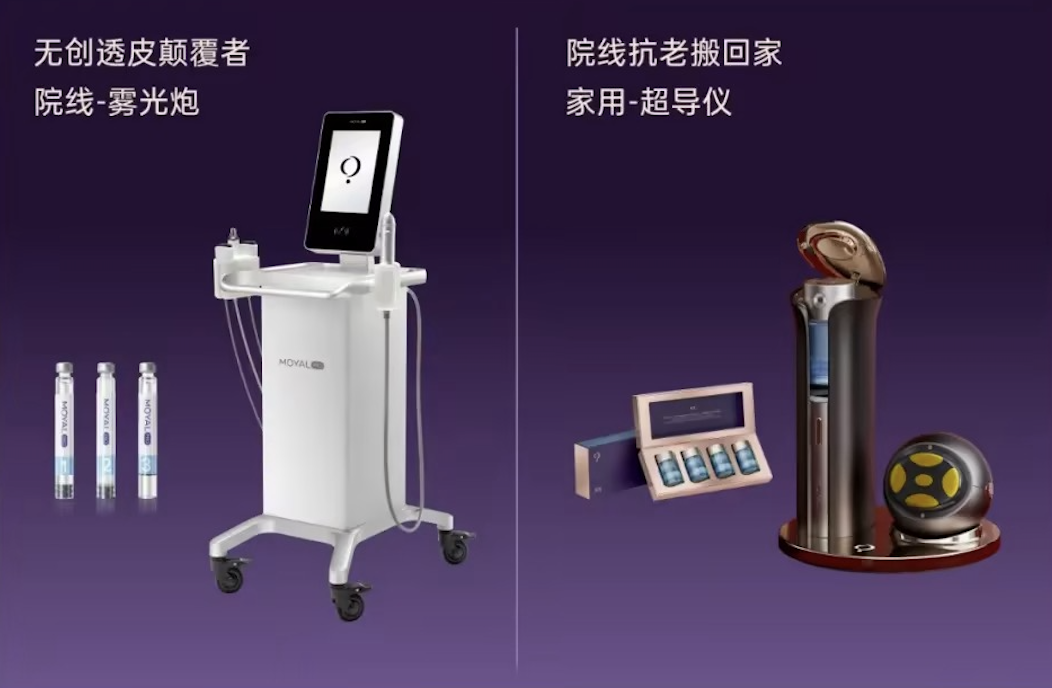
Shenzhen Moer Atomization Health Medical Technology Co., Ltd. (Shenzhen Moer) selected Hezhou High-tech Zone as the site for its factory in July 2024 and signed an investment contract within 20 days, Hezhou Daily and Hezhou News Network reported.
In August, its wholly owned subsidiary, Guangxi Pusimei Health Medical Technology Co., Ltd. (Guangxi Pusimei), was officially established, securing a drug importer registration certificate within 46 days of application.
Currently, the company's production equipment has been moved into the factory building, and production is expected to begin this year, with a potential maximum annual output value of 150 million yuan ($20.7 million).
A report from the January 2025 issue of Guangxi Daily confirmed that Smoore has established its high-end medical equipment project in Hezhou, Guangxi, with an investment aimed at producing atomized beauty devices and related products.
The company signed the investment agreement in September 2024, and factory renovations were completed within 42 days. Production is expected to begin in the first half of 2025, with projected revenue exceeding 1 billion yuan within three years.
Information from Tianyancha shows that Guangxi Pusimei is a wholly-owned subsidiary of Guangdong Pusimei Health Medical Technology Co., Ltd. (Guangdong Pusimei), with Bu Zhiqiang listed as the legal representative.
Guangdong Pusimei, which holds stakes in Guangxi Pusimei, Jiangmen Libao Technology, and Shenzhen Moer, was established in November 2024. Tianyancha shows that these four companies are part of the Pusimei Health enterprise group, with the ultimate controlling entity being Pusimei Hong Kong Limited.
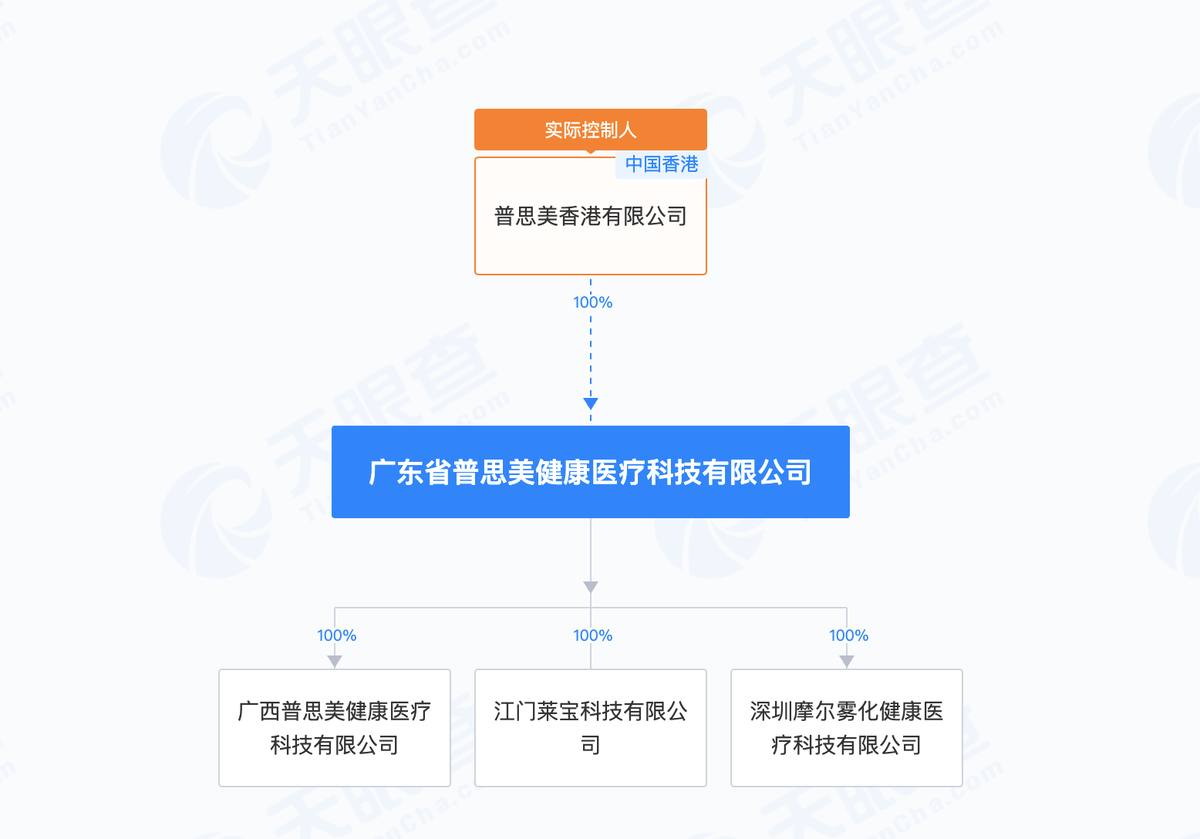
Shenzhen Moer has registered 11 trademarks for the "Lanzhi" brand, including Class 05 (Medicines) and Class 10 (Medical Devices).
The company was established in 2020 under the name Shenzhen Yuejie Neng Electronic Technology Co., Ltd. It was fully acquired by Shenzhen Maxwell Technology Co., Ltd. in 2022, with the ownership of shares changing to Smoore AeroTech Hong Kong Limited in 2023.
On January 7, 2025, all the shares of the company were acquired by Guangdong Pusimei.

Shenzhen MOYAL and China Pharmaceutical University established a joint laboratory on January 9, 2024, to explore innovative applications of atomization technology in the beauty industry.
2Firsts also noticed that MOYAL has signed Chinese actress Zhang Yuqi as the brand ambassador.

We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







