The Swiss Anti-Tobacco Alliance (AT Schweiz) released a survey on March 15 revealing that the majority of disposable e-cigarettes currently being sold in Switzerland are in violation of legal regulations, with the included e-liquid exceeding the allowable limit.
Based on the survey, 2FIRSTS conducted an inventory of the disorder in the Swiss market and conducted an exclusive interview with AT Schweiz.
Excessive E-liquid
During the investigation, AT Switzerland tested the e-liquid content of 11 disposable products. Out of the 11 samples, only one 600-puff ELFBAR product met the standards, while the remaining 10 are suspected of violating regulations. The brands involved include ELFBAR, Vozol Puff Star, and others.
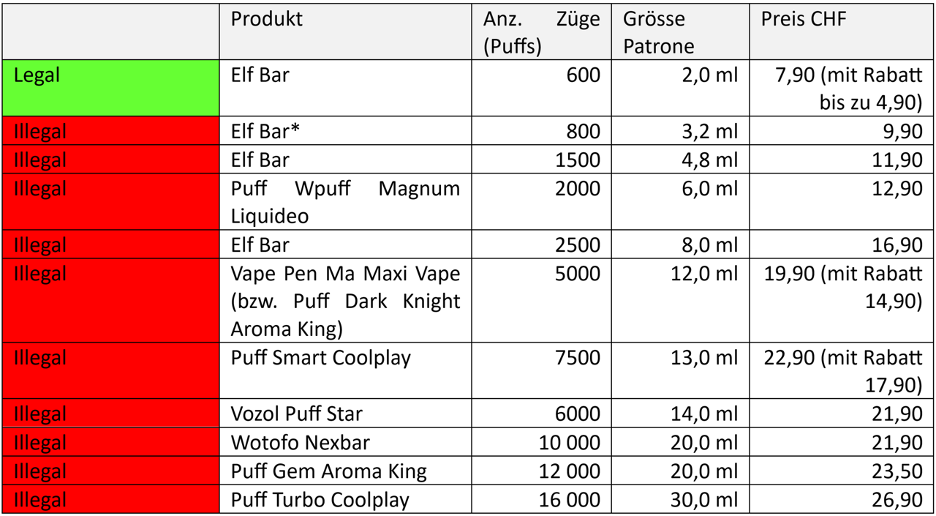
According to the directive issued by the European Union in 2014, there are clear content restrictions on closed e-cigarettes available in the region. The maximum nicotine content in e-liquid is 20 milligrams per milliliter. AT Switzerland sounded the alarm in 2022 regarding the trade of products with illegally high nicotine content of up to 60 milligrams per milliliter; however, these products can still be easily purchased on the internet and in states where such regulations have not been implemented.
As of today, products with a nicotine concentration of 50 mg/ml can still be easily ordered from the website puffbar.eu in Switzerland. It is also convenient to order disposable e-cigarettes directly from online stores in China and have them delivered to your doorstep, seemingly unaffected by restrictions on e-liquid.
E-commerce Giants Chasing High Profits
It is worth noting that during the investigation, AT Schweiz conducted trials and collected evidence from Swiss websites selling e-cigarettes, and found that almost all of the 100 websites surveyed were selling non-compliant products, including the Swiss e-commerce giant Galaxus. On the Galaxus website, a search for "disposable e-cigarette" yields 348 products, with most of them having e-liquid capacities exceeding 2 milliliters.
A banned product is openly being sold on one of Switzerland's largest e-commerce platforms - a situation that is indeed concerning. It is worth noting that the majority of the company's shares are owned by Migros, whose founder Gottlieb Duttweiler's philosophy was to prohibit the sale of tobacco and alcohol.
The prices of these websites are extremely low, making them affordable for young people. For example, the Puff Turbo 16000 is priced at 26.90 Swiss francs on the website wevappy.ch, much lower than traditional cigarettes.
However, even so, the disposable e-cigarette industry in Switzerland is still a lucrative one, with the reason being quite clear. AT Switzerland told 2FIRSTS, "Because the purchase price is being pushed very low": local distributors who purchase goods cheaply from China and resell them at local market prices often make a net profit of 80%-90%. Most of this profit comes from the sale of illegal products. Therefore, even top e-commerce giants cannot resist such temptation.
Weak Law Enforcement
Regarding the illegal commercial activities mentioned above, the Swiss Anti-Tobacco Action Alliance informed 2FIRSTS that, to their knowledge, there has been no precedent in Switzerland for conducting inspections and imposing fines on the sale of non-compliant products.
Switzerland does not have a ready-made market surveillance system. In recent years, the role of tobacco in public health strategies has been gradually marginalized. Previously, the Federal Council's response to MP Lawrence Feierman Ruéller in 2023 was incomplete and there are no signs of systematic checks being implemented.
Switzerland calls on Swiss authorities to quickly ensure that regulations are enforced and to directly present a list of companies operating these websites to state governments. They urge immediate action and demand responses, stating that the results of this investigation will be followed up on and calling for a response from the federal government.
- Immediately recall the substandard products and initiate criminal proceedings against violators.
- The system of import control by the U.S. Customs and Border Protection office.
- Post-market testing, also known as random sampling of products on the market after they have been listed for sale.
- Prohibition of disposable e-cigarettes.
2FIRSTS will continue to monitor AT Schweiz's next steps and the regulatory and compliance developments in the Swiss market.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







