
According to the U.S. Food and Drug Administration (FDA), the FDA has launched a searchable tobacco product database. This is a new user-friendly list that allows individuals to search for tobacco products (including e-cigarettes) legally available for sale in the United States. The database is designed to provide key information to the public, particularly retailers, all in one easily accessible place. This database is updated monthly and can be accessed at www.fda.gov/searchtobacco.
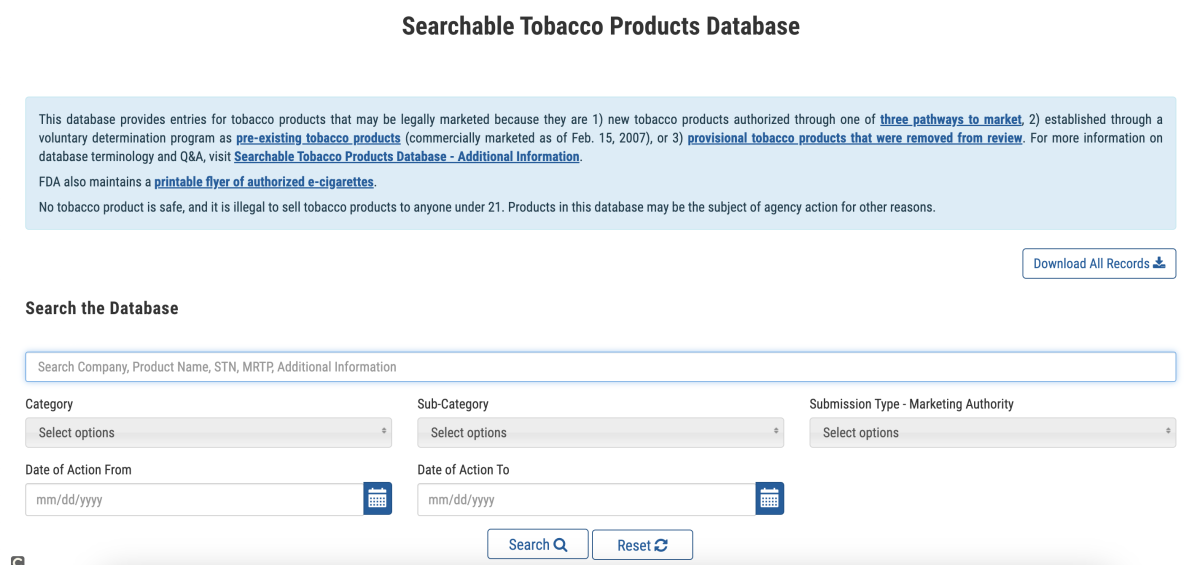
In the database, the FDA provides information on three categories of products:
- New tobacco products authorized for the market through one of three marketing pathways by the FDA;
- Existing tobacco products established through voluntary determinations (on the market since February 15, 2007);
- Temporary tobacco products that have been removed from review.
"The Center for Tobacco Products (CTP) remains committed to providing information that enhances transparency in a useful, timely, and user-friendly manner. We hope this database will become an asset to all parties, including retailers, and will be used to promote compliance with regulations," said Dr. Brian King, Director of the FDA Center for Tobacco Products.
At the time of launch, the database contained nearly 17,000 tobacco products, with over 12,000 being existing tobacco products. For each entry, the FDA provides information about the tobacco product, including the product name, company, category, subcategory, authorized agency for sale in the US, and the date of FDA action.
In addition, the database also includes links to regulatory and scientific documents, such as ordering letters, decision summaries, as well as environmental assessments (EAs) and related documents associated with tobacco product applications.
In order to help explain some terminology and background information of the database content, the FDA has also developed a searchable Tobacco Product Database - Additional information webpage. This webpage also includes answers to potential questions about the database, including questions about unauthorized products pending applications. Generally, the FDA cannot provide information about pending applications to protect proprietary information. Additionally, for new products that require authorization, a pending application does not mean that the product can be safely marketed.
The launch of this public database is part of the center's ongoing commitment to providing resources for regulated industries and clearly communicating agency actions to the public. Other recent activities aimed at enhancing transparency include the listing of activities in the center's quarterly updates on evaluations conducted by the Reagan-Yudell Foundation.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







