Key points
- Expert views: Clinicians and researchers say “vitamin diffusers/wellness vapes” may contain undeclared chemicals; heat can create unknown breakdown products; no evidence supports health benefits from inhaled vitamins.
- Enforcement: On Aug 19, HSA fined a man found using a vitamin diffuser outside the State Courts; buying, using, or possessing imitation tobacco products is an offence, with fines of up to S$2,000.
- Risk reminder: Experts note past harms from inhaled unknowns, including the 2019 U.S. EVALI outbreak.
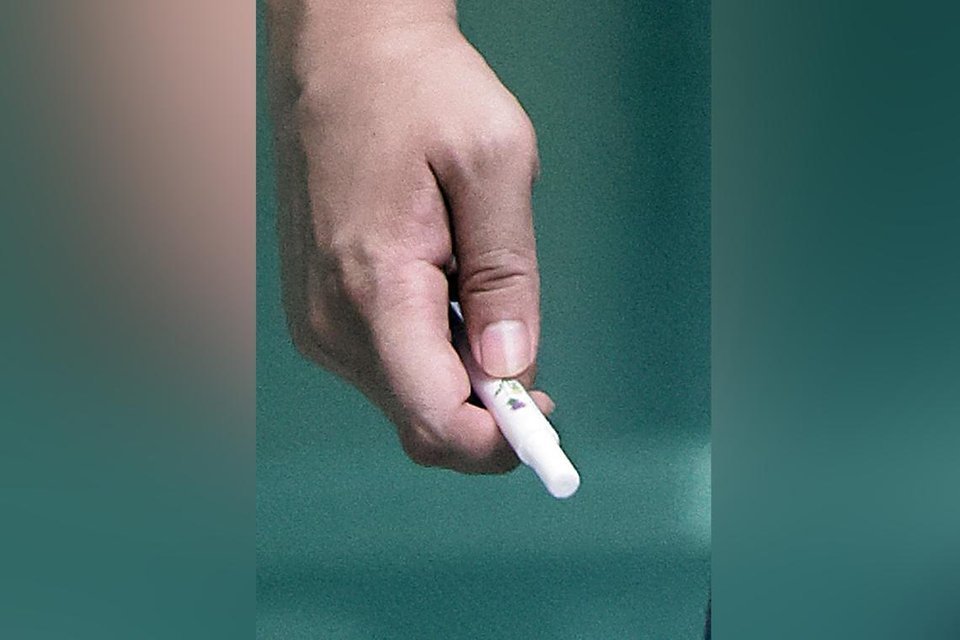
2Firsts, September 4, 2025 — Online sellers are promoting “vitamin diffusers” and “vitamin sticks” as a wellness-oriented alternative to e-vaporisers, a trend seen in the UK, Australia, and the United States. Influencer marketing claims these devices can deliver caffeine, vitamin B12, essential oils, or melatonin via inhalation.
Respiratory physician Dr Philip Eng said that history shows inhaling unknown substances can make people ill. Beyond the established risks of tobacco smoking, he pointed to the U.S. EVALI outbreak in 2019. He added there is no medical evidence to support the purported benefits of vitamin diffusers and urged caution before ingesting or inhaling any substance.
Professor Eric Chan, deputy head of research at the National University of Singapore’s Department of Pharmacy, said substances considered safe orally (such as vitamin B12) may still pose risks in vaping devices if other chemicals are present. He cited a 2024 toxicology study in the U.S. on melatonin-laced vape products that detected contaminants, including pharmaceuticals and industrial chemicals. Without health-authority oversight and full ingredient disclosure, undeclared chemicals raise safety concerns, he said. Heat may also degrade vitamins, melatonin, and contaminants into new compounds with unknown safety profiles. Vitamins and supplements, he noted, should be used under medical advice for confirmed deficiencies.
Dr Puah Ser Hon, head of Respiratory and Critical Care Medicine at Tan Tock Seng Hospital, said there is no evidence that inhaled vitamins provide health benefits. Marketing such products as “healthy,” he warned, is misleading and may pose significant risks to respiratory and overall health. He added that device chemicals and the effects of inhalation are not yet fully understood.
HSA stated that although a vitamin diffuser is not an e-vaporiser, it is still an offence under the Tobacco (Control of Advertisements and Sale) Act to purchase, use, or possess imitation tobacco products. Such products are banned because they mimic smoking via devices that may appear harmless, particularly to younger, vulnerable users. Beyond the known risks of inhaling substances not intended for consumption, these items may act as a gateway to cigarettes or even hard drugs.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com









