
On December 19, 2024, FDA announced the issuance of warning letters to eight online retailers and one manufacturer for selling and/or distributing unauthorized flavored, disposable e-cigarettes. Some of the unauthorized products cited in the warning letters are marketed under brand names popular among youth, including Geek Bar and Lost Mary. Other unauthorized products cited feature the names and/or images of celebrities.
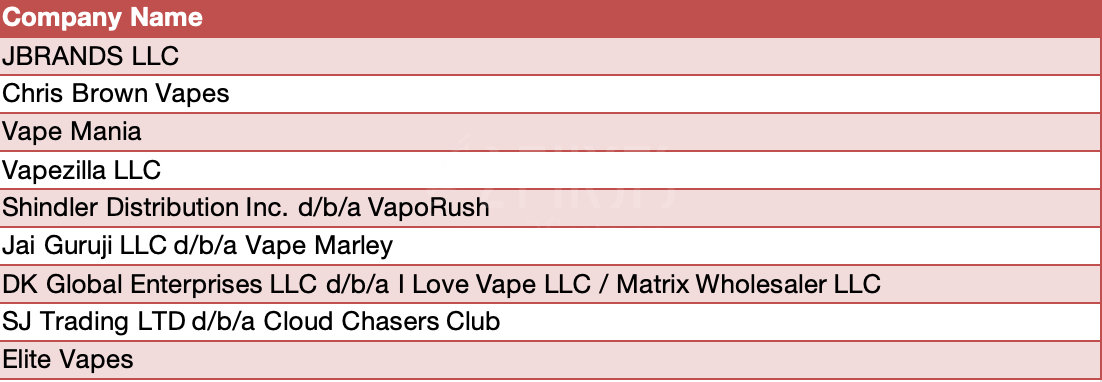
The firms receiving these warning letters sold and/or distributed e-cigarettes in the United States that lack authorization from FDA to be legally marketed in the U.S., which is in violation of the Federal Food, Drug, and Cosmetic Act. In addition to the violations mentioned in the warning letters, the firms were warned to address any violations that are the same as, or similar to, those stated in the warning letter and to promptly take necessary actions to comply with the law. Failure to promptly correct the violations can result in additional actions such as an injunction, seizure, and/or civil money penalty.
FDA is committed to enforcing the law, and the agency will continue to work with its federal enforcement partners, as needed, to address unauthorized tobacco products in the United States. This latest round of warning letters demonstrates FDA’s continued efforts to remove unauthorized e-cigarette products from the market, particularly those that appeal to youth. To date, FDA has issued more than 700 warning letters to firms for manufacturing, selling, and/or distributing unauthorized new tobacco products, issued more than 800 warning letters to retailers for the sale of unauthorized tobacco products, and filed civil money penalty complaints against more than 83 manufacturers and more than 175 retailers for distribution and/or sale of unauthorized tobacco products.
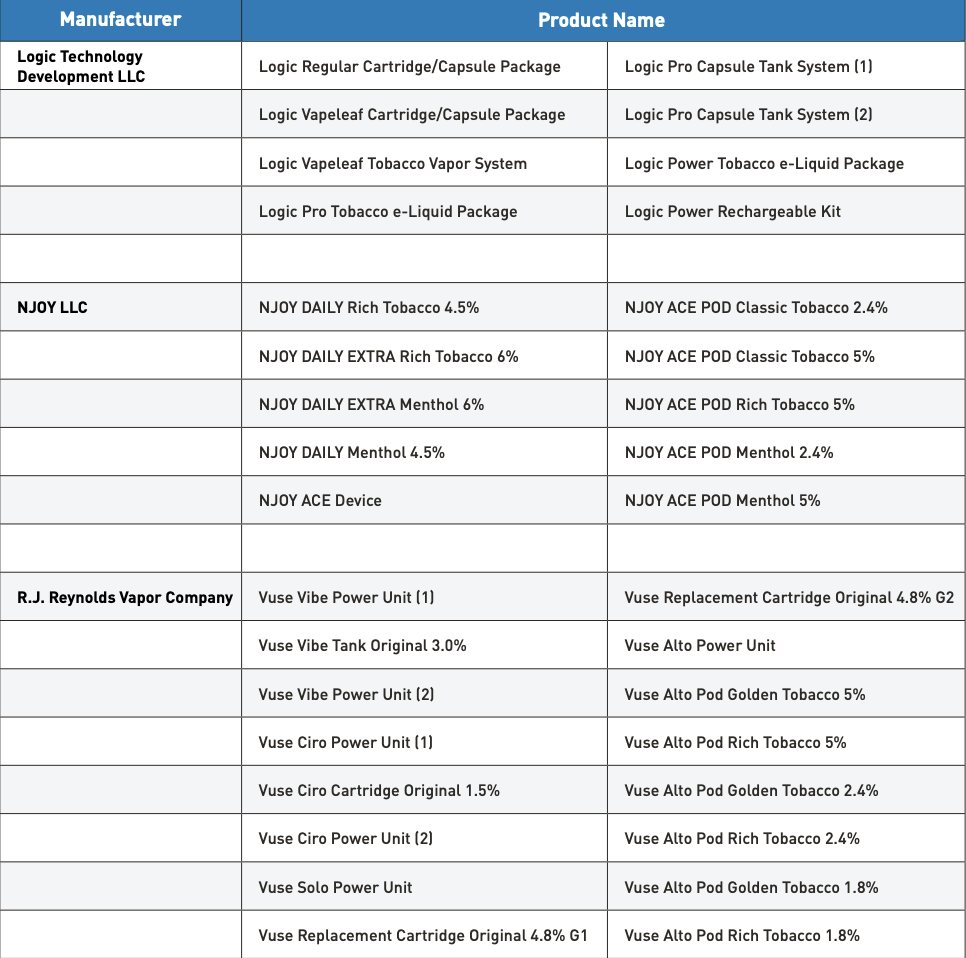
As of December 19, 2024, FDA has authorized 34 e-cigarette products and devices. The agency maintains a printable one-page flyer of all authorized e-cigarette products that retailers can consult to determine which products may be lawfully marketed and sold in the United States. Entities manufacturing, importing, selling, or distributing e-cigarettes without the required premarket authorization risk enforcement.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







