
Special Notice:
1.This article is for internal industry communication only and does not make any recommendations for brands or products.
2.The images presented in this article are intended solely for factual description and are not to be used as advertisements for any product.
3.Minors are prohibited from accessing this article.
Product highlights:
Integrated pod system: The pod part of SKE BAR is integrated with the outer shell, and the rechargeable battery can be reused.
Environmentally friendly materials: SKE BAR e-cigarette uses recyclable PCTG material.
Compliance products: SKE BAR has been publicized in the UK Medicines and Healthcare products Regulatory Agency and is available on various e-cigarette distributors' websites in the UK.
Diverse flavors: Offering 35+ flavor SKUs, mainly focusing on "fruit + ice" flavors.
The e-cigarette brand SKE recently launched multiple new products on its official website, including small-capacity pod systems and hookah-style e-cigarettes. Among them, the new small-capacity pod system - SKE BAR has been launched on some e-cigarette distributors' websites in the UK. 2Firsts analyzed the product information of SKE BAR e-cigarette.
Parameter information: The parameters of the disposable e-cigarette SKE Crystal Bar are basically the same
Previously, SKE had launched its signature disposable e-cigarette, the SKE Crystal Bar. The newly released SKE BAR e-cigarette has a design that is very similar to the SKE Crystal Bar. 2Firsts has conducted a detailed comparison of the specifications of these two products, with specific information shown in the table below.
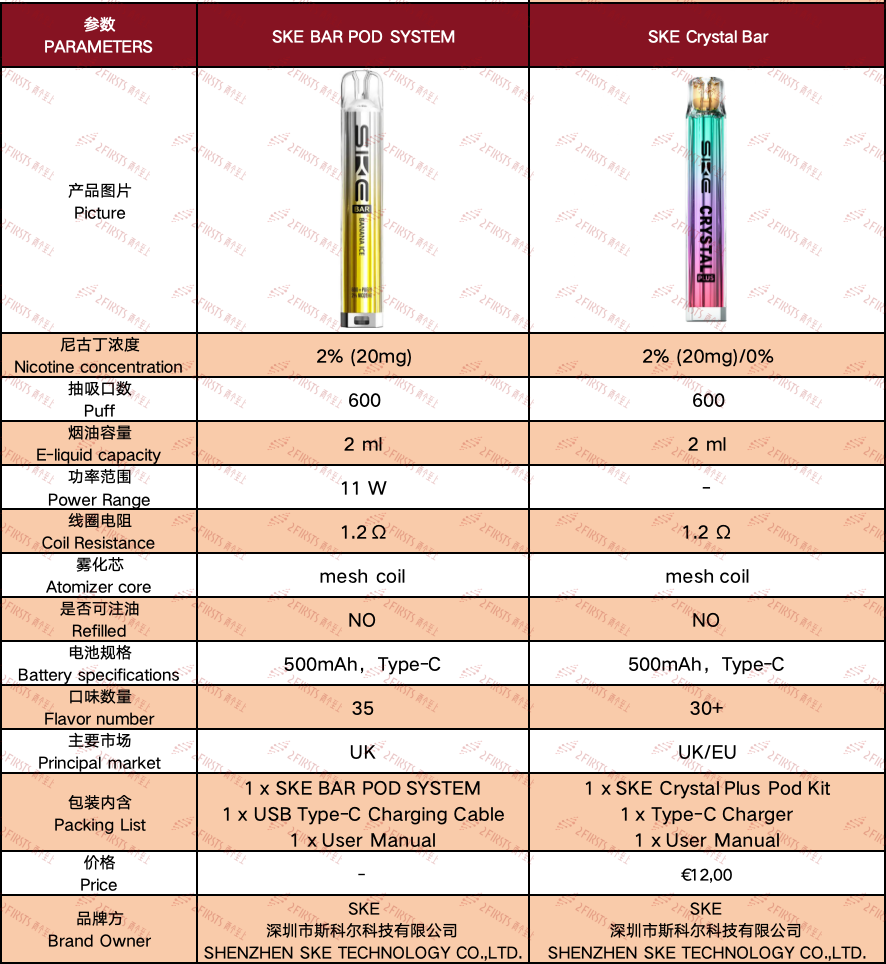
In terms of specifications, the disposable e-cigarette SKE Crystal Bar and the pod system SKE BAR are consistent in terms of puff count, e-liquid capacity, coil resistance, atomizer core, and battery specifications; the main difference between the two lies in the different product forms they take.
It is worth noting that in the past, SKE has released products using the naming convention of "SKE Crystal+", but recently, several new products from SKE have not included the term "Crystal" in their names.

Product feature: Uses an integrated, interchangeable pod system
According to the official website of the SKE brand, the SKE BAR uses "the all-in-one replaceable pod system", which is an integrated replaceable pod system. From the images, it can be seen that the pod part of the SKE BAR is designed integrated with the product shell. The official website states that this part is made of "recyclable PCTG material", while the rechargeable battery can be reused.

According to the EU Battery Regulation, all portable devices, including disposable e-cigarettes, must be equipped with easily removable and replaceable batteries by 2026. The design of the SKE BAR e-cigarette is likely intended to comply with this upcoming regulation. Currently, multiple brands, including INNOKIN Trine, have already introduced products that meet this requirement (for more information, please read "Innokin Trine: A Revolutionary Rechargeable E-Cigarette with Replaceable Battery").
Compliance information: Already publicly disclosed by the UK Medicines and Healthcare Products Regulatory Agency in March
2Firsts searched the e-cigarette search system of the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette notification system found that the product "SKE BAR" was submitted for public notice by SHENZHEN SKE TECHNOLOGY COMPANY LIMITED, also known as Sikary, on March 6th, 2025.
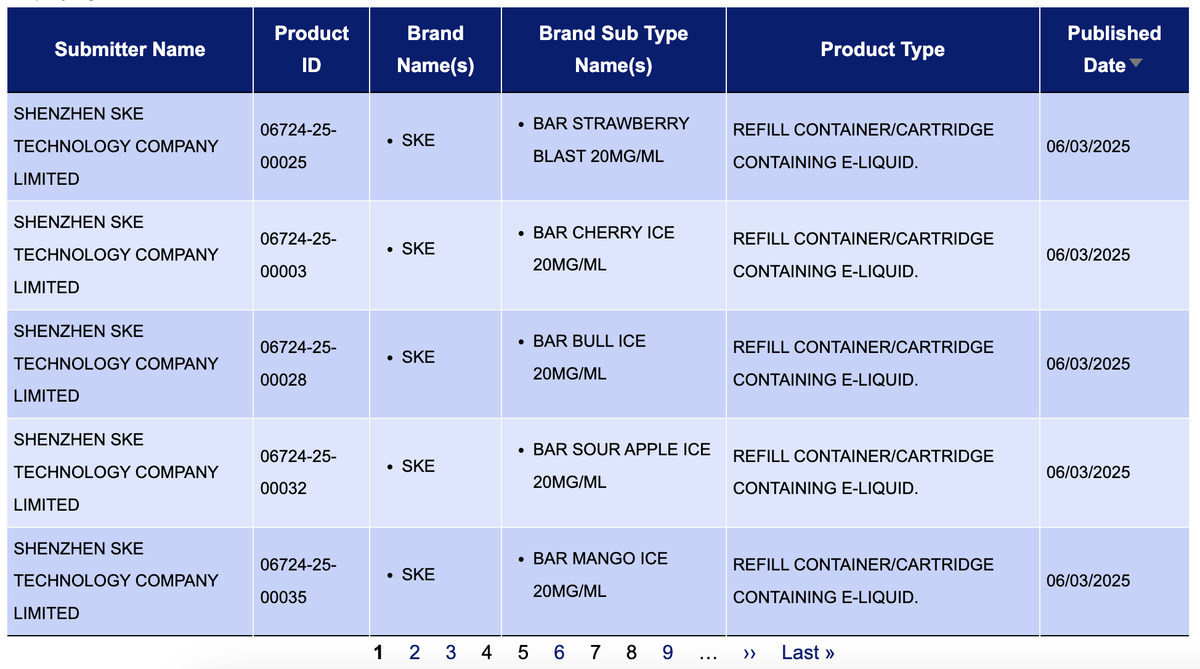
This batch includes a total of 36 SKUs, with products mainly flavored as "fruit + ice" and a nicotine concentration of 20MG/ML.

Sales market: Primarily targeting sales in the United Kingdom
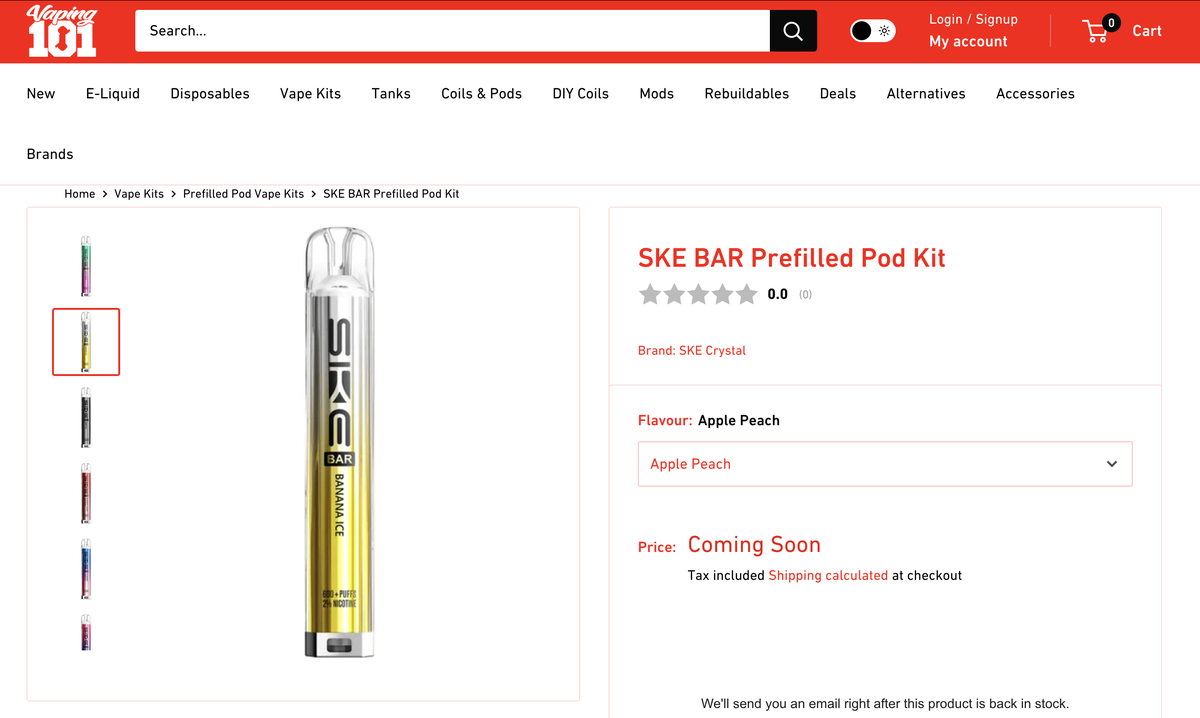
Currently, the SKE BAR has landed on the SKE brand official website and various e-cigarette distributor websites in the UK such as vapeclub and vaping101. The product status is displayed as "Coming Soon" and the product price has not been released yet.
2FIRSTS focuses on providing readers with the latest product information in the tobacco industry. We welcome readers to submit articles to us and share the newest products in the e-cigarette sector.
If you have any unique insights or information, please feel free to contact us at info@2firsts.com, or reach out to 2Firsts CEO Alan Zhao on LinkedIn at any time.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







